A symbolic milestone was passed today when the 1000th new clinical trial for COVID-19 was listed, as reported by Ancora.ai. Clinical trials represent the safest and most effective way to generate scientific evidence on prospective testing, treatment and prevention strategies to combat Coronavirus.
Zurich, Switzerland – April 30, 2020 /MarketersMedia/ —
A symbolic milestone was passed today when the 1000th clinical trial for COVID-19 was listed on clinicaltrials.gov, the global public database for clinical trial registry. Clinical trials to study Coronavirus strategies quickly began to be initiated in January 2020 and have been increasing exponentially in number since.
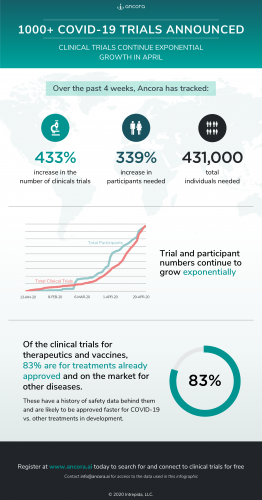
Ancora.ai, a new digital health product for clinical trial search and match, has tracked a 433% increase in the number of clinical trials listed for COVID-10 in the past month alone. These trials need to recruit more than 430,000 participants to be fully enrolled and completed.
Healthcare systems are overwhelmed due to the pandemic and have little spare time or resource to devote to recruiting for and conducting clinical trials. However, clinical trials represent the safest and most effective way to generate scientific evidence on prospective testing, treatment and prevention strategies to combat Coronavirus.
Ancora.ai was launched in March 2020 by Intrepida, a Swiss technology company focused on AI applications for healthcare, in recognition of the critical need to support Coronavirus clinical research.
Intrepida’s CEO, Danielle Ralic, said of the milestone 1000th trial: “It is truly impressive how quickly the global clinical research community has responded to the pandemic. Ancora.ai has posted 567 COVID-19 trials that we can connect patients and healthy volunteers to, and we are updating the site on a daily basis to provide the latest information to the public as the numbers are changing so swiftly.”
Of the clinical trials announced so far for therapeutics and vaccines, 83% are for treatments already approved and on the market for other diseases. These have a history of safety data behind them and are likely to be approved faster for COVID-19 vs. other treatments in development.
Ralic commented, “Users can register for free on Ancora.ai to search for and connect to COVID-19 trials, as a patient or a healthy volunteer, and by doing this they stand to gain early access to innovative treatments and new vaccines for the virus, which is a benefit of research participation that not many people are aware of.”
About Intrepida LLC
Intrepida LLC, founded in 2017, is focused on analytics and building artificial intelligence (AI) applications for healthcare challenges. Based in Zurich, Switzerland, the company’s mission is to improve healthcare using technology. Intrepida built Ancora.ai to help connect patients with the latest innovations in medical research, while helping trial sponsors recruit as quickly and efficiently as possible, in order to reduce delays in research, as delays in research stop new treatments from reaching patients who need them. In addition to COVID-19, Ancora.ai supports oncology indications, including breast cancer, lung cancer and cervical cancer. Cofounded by three former colleagues, Intrepida’s team has a 50/50 gender balance and includes data scientists, technologists and scientists who all share deep expertise in the healthcare industry. Intrepida is a member of NVIDIA’s Inception Program, Startup with IBM, Almirall’s Digital Garden, the Barcelona Health Hub, the Future Perfect accelerator for COVID-19, and has participated in the Google for Startups Female Founders Program.
Contact Info:
Name: Emily Jordan
Email: Send Email
Organization: Intrepida
Address: Pfingstweidstrasse, 8005 Zürich, Switzerland
Phone: +49 1778405531
Website: http://www.ancora.ai
Video URL: https://www.youtube.com/channel/UCBWHw4VShOAreUcHkD77Vdw/videos
Source: MarketersMedia
Release ID: 88955676
