High prevalence of target diseases, unhealthy lifestyle, product launches, increasing strategic developments such as partnerships and agreements are key factors contributing to high CAGR of In-Vitro Diagnostics (IVD) Quality Control Market during forecast period.
New York City, United States – June 27, 2020 /MarketersMedia/ —
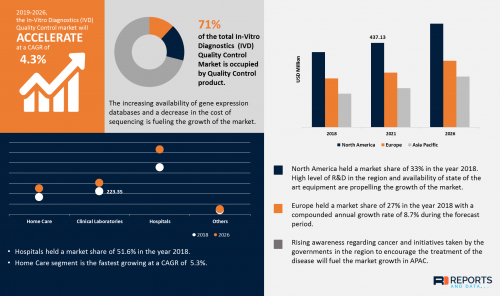
According to the current analysis of Reports and Data, the global In-Vitro Diagnostics (IVD) Quality Control Market was valued at USD 881.8 Million in 2018 and is expected to reach USD 1229.3 Million by year 2026, at a CAGR of 4.3%.
IVD quality controls are tests used to authenticate the consistency of IVD testing system to confirm precision of test findings and assess the influence of factors such as environmental conditions and operator’s performance on test results. Key recent developments: in June 2019, Illumina, Inc. announced the launch of VeriSeq NIPT Solution v2, a CE-IVD; next-generation sequencing (NGS)-based approach to noninvasive prenatal testing (NIPT), delivers the most comprehensive view of the fetal genome compared to other CE-IVD NIPT products.
This is the latest report covering the current COVID-19 scenario. The coronavirus pandemic has greatly affected every aspect of the worldwide industry. It has brought along various changes in market conditions. The rapidly changing market scenario and initial and future assessment of the impact are covered in the research report. The report discusses all the major aspects of the market with expert opinions on the current status along with historical data.
Get a sample PDF copy of the report @ https://www.reportsanddata.com/sample-enquiry-form/1508
Key players mentioned in the research report are:
Agilent Technologies, Abbott Diagnostics, Becton, Dickinson and Company, BioMerieux, Inc., Bio-Techne, Bio-Rad Laboratories, Inc., DiaSorin, Fortress Diagnostics, Future Diagnostics Solutions, Helena Laboratories, Hologic, Inc., Illumina, Laboratory Corporation, Quidel Corporation, Roche Diagnostics, Randox Laboratories Ltd., Seracare Life Sciences, Inc., Sun Diagnostics, LLC., Sero AS, Sysmex Corporation, Surmodics, Inc., Siemens Healthcare GmbH, Technopath Clinical Diagnostics, and Thermo Fisher Scientific, Inc.
In terms of applications, the global In-Vitro Diagnostics (IVD) Quality Control market can be segmented into:
Clinical Chemistry
Immunochemistry
Haematology
Molecular Diagnostics
Coagulation/Haemostasis
Microbiology
Others
In terms of types, the global In-Vitro Diagnostics (IVD) Quality Control market can be segmented into:
Quality Controls
Data Management Solutions
Quality Assurance Services
To get a discount on the report, click @ https://www.reportsanddata.com/discount-enquiry-form/1508
For geographical segmentation, regional supply, application-wise, and type-wise demand, key players, and others, this report covers the following regions: North America, Europe, Asia-Pacific, South America, and Middle East & Africa. The report sheds light on the competitive landscape of the market that covers the product offerings, services, market shares, and business overview. This In-Vitro Diagnostics (IVD) Quality Control Market research report covers various dynamic aspects like the market drivers, restraints and challenges, and growth prospects. The prominent and leading companies are profiled in the report.
Further key findings from the report suggest:
• The American Clinical Laboratory Association (ACLA) states that more than 7.5 billion lab tests are performed in U.S. annually and 80% of clinical decisions are taken after lab testing
• In December 2018, BioMérieux announced its culture bottles BACT/ALERT® BPA and BPN have received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for quality control testing of leukocyte-reduced apheresis platelet (LRAP) units with the BACT/ALERT® VIRTUO® fully automated blood culture system
• In-Vitro Diagnostics (IVD) Quality Control Market is fastest growing at a CAGR of 3% in Asia Pacific owing to rising incidence of IVD tests in developing countries such as India and China due to upsurge in number of clinical laboratories, global awareness among patients
• FDA is encouraging convergence of regulatory systems for medical devices, which is anticipated to boost trade, while protecting public health through regulatory means
• Data Management Solutions segment is accounted to be the second-leading segment which is valued at USD 155.6 million because it gives access to LIS and easy for managing test results of patients and even popular due to dependable laboratory outcomes
Get the full report description, TOC, Table of Figures, Charts @ https://www.reportsanddata.com/report-detail/in-vitro-diagnostics-ivd-quality-control-market
Thank you for reading our report. For further details or to inquire about customization, please let us know and we will offer you the report as per your needs.
About Us:
Our in-house experts assist our clients with advice based on their proficiency in the market that helps them in creating a compendious database for the clients. Our team offers expert insights to clients to guide them through their business ventures. We put in rigorous efforts to keep our clientele satisfied and focus on fulfilling their demands to make sure that the end-product is what they desire. We excel in diverse fields of the market and with our services extending to competitive analysis, research and development analysis, and demand estimation among others, we can help you invest your funds in the most beneficial areas for research and development. You can rely on us to provide every significant detail you might need in your efforts to make your business flourish.
Contact Info:
Name: John Watson
Email: Send Email
Organization: Reports And Data
Address: 40 Wall St. 28th floor New York City, NY 10005 United States
Phone: +1-212-710-1370
Website: https://www.reportsanddata.com/
Source: MarketersMedia
Release ID: 88965970
